Abstract
Thrombocytopenia refers to the sum of a series of clinical diseases with a platelet count of less than 100×109/L in peripheral blood, including primary immune thrombocytopenia (ITP), tumor chemotherapy-related thrombocytopenia (CIT), infection, etc. The traditional treatments of thrombocytopenia are not always effective and often lead to serious side effects. Therefore, thrombopoietin (TPO) and TPO receptor agonist (TPORA) have been found to play a role as early recombinant thrombopoietin and gradually become a new alternative treatment. At present, there are few studies on the treatment of thrombocytopenia with Avatrombopag, as a second-generation TPORA, at home and abroad.
We designed a prospective, single arm, exploratory clinical study to evaluate the efficacy and safety of Avatrombopag in the treatment of thrombocytopenia with TPO or TPORA ineffective. The patients with platelet counts less than 25×109/L who have failed to respond to TPO or other TPORA (including Eltrombopag,Herombopag) treatment in the Fourth Affiliated Hospital of Medical College of Zhejiang University were recruited. All the eligible patients were administered oral Avatrombopag at an initial dose of 20mg/d or 40 mg/d, monitored complete blood count at least once a week, and adjusted the drug dose according to the platelet level. The efficacy and safety of the drug were analyzed by monitoring the changes of platelet level and bleeding grade within 4 weeks after treatment, and the adverse reactions of Avatrombopag. This study was registered at chictr.org.cn (ChiCTR2200061853).
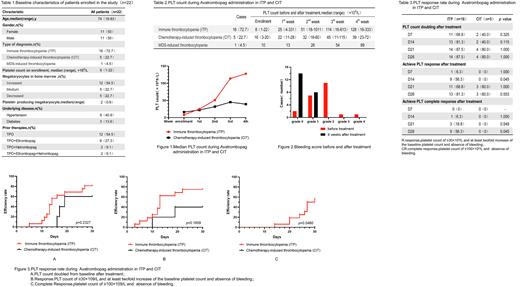
22 patients were enrolled in the clinical trial, aged from 18 to 83 years, with a median age of 74 years, including 16 patients with immune thrombocytopenia (ITP), 5 patients with chemotherapy-induced thrombocytopenia (CIT) and one patient with thrombocytopenia caused by MDS. Baseline characteristics of patients enrolled in the study are shown in Table 1. During 4-w Avatrombopag treatment period, the median platelet count in ITP group was higher than that in CIT group (Table 2 and Figure 1). 20 patients (90.9%) had bleeding symptoms at baseline, which decreased to 36.4% (8/22) in the end of follow-up (Figure 2). After 4 weeks of treatment, 17 patients showed response to the drug (platelet count between 30 and 100×109/L, at least doubling of the baseline count, and absence of bleeding), with a median response time of 14 (5-27) days. Among them, 13 patients were ITP, 3 patients were CIT, and 1 patient was MDS-induced-thrombocytopenia. The difference was not statistically significant (p=0.654). 9 patients achieved complete response (platelet count higher than 100×109/L and absence of bleeding), all of them were ITP. Compared with CIT patients, the difference was statistically significant (p=0.045) (Table 3 and Figure 3). 20 of 22 patients (90.9%) no longer required platelet transfusion at the study end point. During the administration period, one patient was found to have mild liver dysfunction that is difficult to explain by other diseases, and the other patients were not found to have drug-related adverse effects.
In conclusion, Avatrombopag is an effective and safe drug for the treatment of patients with relapse and refractory thrombocytopenia. Compared with CIT patients, Avatrombopag have faster response to platelets and higher remission rate for ITP patients.
he research was supported by the Key R&D Program of Zhejiang, No. 2022C03137; Public Technology Application Research Program of Zhejiang, China, No. LGF21H080003; the Key Project of Jinhua Science and Technology Plan, China, No. 2020-3-011; the 2019-2022 Key Medical Discipline (Hematology) Fund of Jinhua, China.
Correspondence to : Dr Jian Huang, Department of Hematology, The First Affiliated Hospital of Zhejiang University School of Medicine.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal